Bcs Classification System
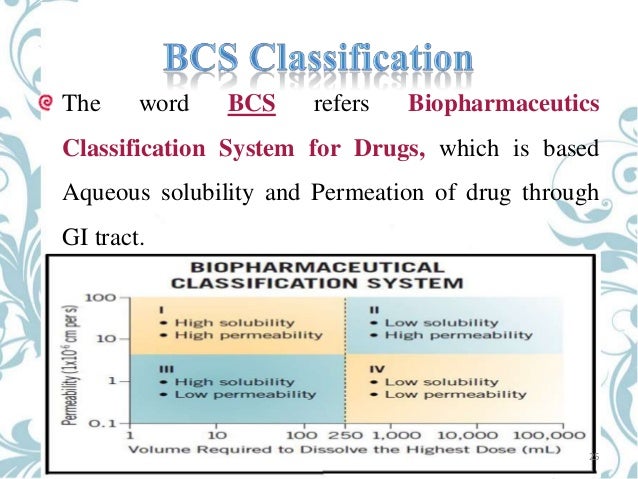
The Biopharmaceutics Classification System (BCS) is the result of continuous efforts in mathematical analysis for the elucidation of the kinetics and dynamics of the drug process in the gastrointestinal tract (GIT) for NDA (New Drug Application) and ANDA (Abbreviated New Drug Application) filings and biowaivers. Employee handbook sample. The BCS classification system is used to categorize drugs and serves to help anticipate whether drugs will have bioavailability/ bioequivalence problems. BCS classifies drugs according to their solubility and permeability. The Biopharmaceutics Classification System (BCS) and Biopharmaceutics Drug Disposition Classification System (BDDCS) have classified compounds according to their extent of solubility (high or low) using the rate and the extent of dissolution ( Amidon et al., 1995, Yu et al., 2002) and/or dose number ( Benet et al., 2011, Dahan et al., 2013 ).
Bcs Class 1 Drugs
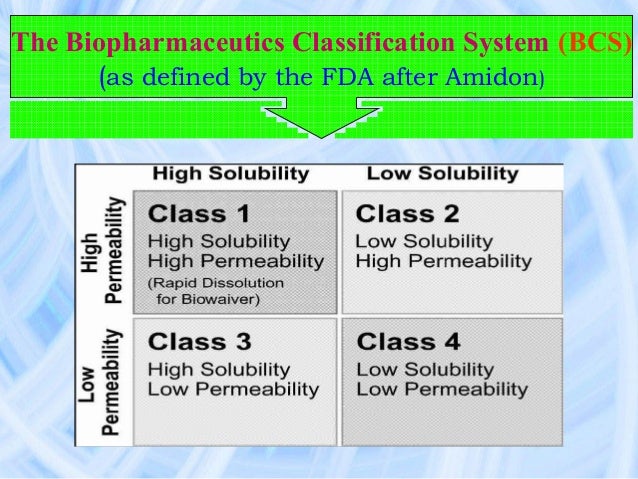
Applying for a BCS-based biowaiver is restricted to highly soluble drug substances with known human absorption and considered not to have a narrow therapeutic index. The concept is applicable to immediate release, solid pharmaceutical products for oral administration and systemic action having the same pharmaceutical form.

Bcs Classification System
However, it is not applicable for sublingual, buccal, and modified release formulations. For orodispersible formulations the BCS-based biowaiver approach may only be applicable when absorption in the oral cavity can be excluded. Biowaiver may be applicable when the active substance(s) in test and reference products are identical. Biowaiver may also be applicable if test and reference contain different salts provided that both belong to BCS-class I. Biowaiver is not applicable when the test product contains a different ester, ether, isomer, mixture of isomers, complex or derivative of an active substance from that of the reference product, since these differences may lead to different bioavailabilities not deducible by means of experiments used in the BCS-based biowaiver concept. Investigations related to the medicinal product should ensure immediate release properties and prove between the investigative products, i.e. Test and reference show similar in vitro dissolution under physiologically relevant experimental pH conditions.



